For chronic rhinosinusitis (CRS)
TIME TO EXHALE
with XHANCE
Experience what relief could feel like with a different kind of treatment
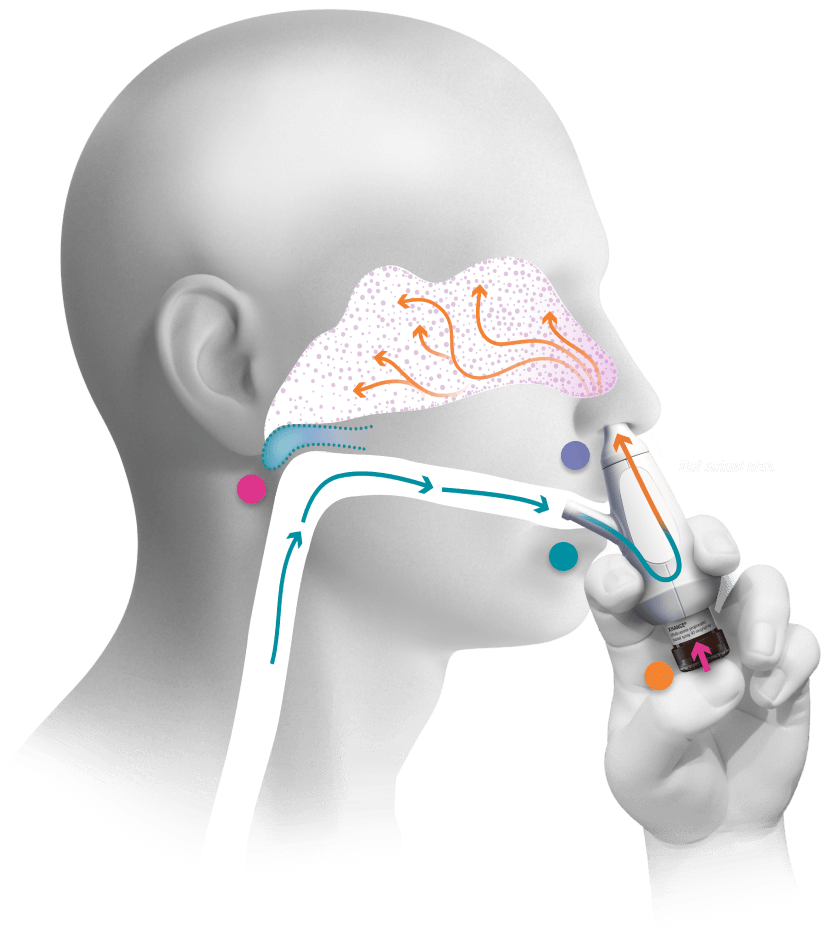
Exhaling helps deliver the medicine to where it is needed
- Designed to drive the medicine deep into the nasal cavity, the site of inflammation in chronic rhinosinusitis
- Closes a structure in the back of the mouth called the soft palate, which helps prevent drainage of the medicine into the throat
- Press the bottle to activate the spray
- Soft palate is sealed closed when you exhale
- Medicine is released into your airflow
- Flexible mouthpiece

- Designed to drive the medicine deep into the nasal cavity, the site of inflammation in chronic rhinosinusitis
- Closes a structure in the back of the mouth called the soft palate, which helps prevent drainage of the medicine into the throat
- Press the bottle to activate the spray
- Soft palate is sealed closed when you exhale
- Medicine is released into your airflow
- Flexible mouthpiece
XHANCE has proven results to help you move forward
XHANCE helped improve the primary symptoms of chronic rhinosinusitis

Nasal
congestion

Facial pain
or pressure

Runny nose and
postnasal drip

Reduced or loss
of sense of smell
In recent studies, some people using XHANCE experienced a reduction in symptom flare-ups* through 24 weeks.
*Flare-ups are a worsening of symptoms requiring antibiotics or an acute care visit.
Do not use XHANCE if you are allergic to fluticasone propionate or any of the ingredients in XHANCE.
How to take XHANCE: Seal, Aim, Blow, Press
Prime XHANCE before first use. Remove the cap and shake away from your face and eyes. With the cap removed, keep the bottle away from your face and pump 7 times, or until you see a fine mist. If you have not used XHANCE for 7 or more days, re-prime by spraying 2 times before use.

STEP 1: SEAL
First, shake. Then, place your fingers on the indented grip BELOW the mouthpiece.
Do not place your fingers above or over the flexible mouthpiece.
Gently insert the nosepiece into your nose and the flexible mouthpiece into your mouth.
Keep a tight seal between the nosepiece and your nostril.
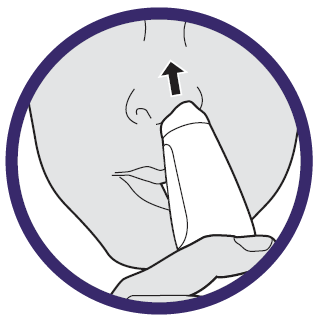
STEP 2: AIM
Aim the device upward between your eyes.

STEP 3: BLOW
Take a deep breath and blow hard into the mouthpiece.
Imagine you are blowing up a balloon.
Blow, don’t sniff, and don’t block your other nostril.

STEP 4: PRESS
Keep blowing hard as you press the bottle with your thumb, releasing the medicine deep into your nasal passages.
Repeat these steps for your other nostril.
DID I DO IT RIGHT?
- Shake before each use
- Keep a snug fit in nose and mouth
- Continue blowing hard while pressing the bottle
- Do not sniff, inhale, or block second nostril
- Administer only the prescribed number of sprays in each nostril
FOR BEST RESULTS
- Use XHANCE regularly, twice daily. Follow your doctor’s instructions, with either one or two sprays per nostril
- Do not take more than your prescribed dose of XHANCE each day
- Remember to refill your prescription
monthly or as prescribed by your doctor
If you have questions about how to use XHANCE, refer to the Instructions for Use.
If your commercial health insurance covers XHANCE, your out-of-pocket cost
could
be as little as $0 using the XHANCE copay card

Eligible, commercially insured patients can pay as little as $0 for their prescription of XHANCE.†
Download copay card >Be sure to download or print your XHANCE
Copay Card to receive available
savings.
†Terms and conditions apply. Patients must be commercially insured. May not be used if the patient is enrolled in a government-funded prescription insurance program.
